introduction of brazing
Views Send Enquiry
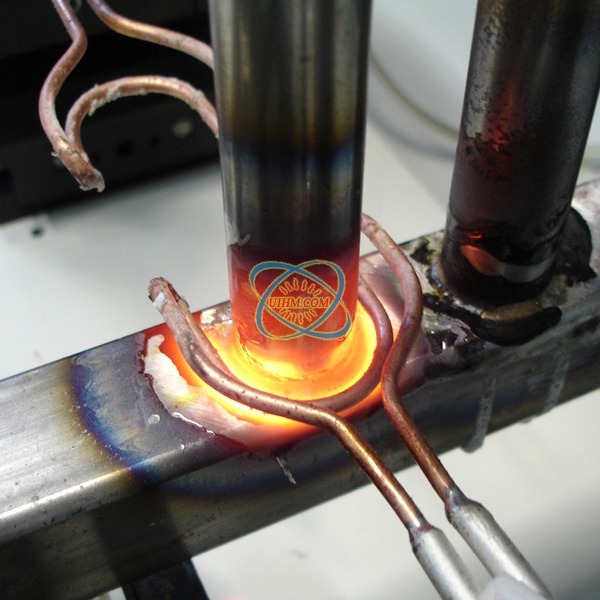
What is brazing?
The term “brazing” can be applied to any process which joins metals (of the same or dissimilar composition)
through the use of heat and a filler metal with a melting temperature above 840° F (450° C), but below the melting point of the metals being joined. In furnace brazing, temperatures of 2050° to 2100° F (1120° to 1150° C) and above are not uncommon, especially when brazing stainless steels with nickel-based filler metals or carbon steel with copper filler metal. Other very high temperature brazing applications include molybdenum with pure nickel as the filler metal and cobalt with a cobalt alloy filler metal.
A successfully brazed joint often results in a metallurgical bond that is generally as strong or stronger than the base metals being joined. Modern brazing technology has extended the definition to include the bonding of metal to non-metallic substrates, including glass and refractory materials. However, this publication is limited to brazing of metals only,and, specifically, furnace brazing of metals.
How does brazing join materials? In furnace brazing, the parts or assemblies being joined are heated to the melting point of the filler metal being used. This allows the molten filler metal to flow via capillary action into the closefitting
surfaces of the joint and to form an alloy of the materials at the transition point upon solidification.
The base metals do not melt, but they can alloy with the molten filler metal by diffusion to form a metallurgical bond.
Because the metallurgical properties at the brazed joint may differ from those of the base metals, the selection of the appropriate filler metal is critical.
Depending on the desired properties of the application, the brazing operation can be used to impart a leaktight seal and/or structural strength, with excellent appearance characteristics, in addition to joining for the purpose of extending section length,e.g., in piping or tubing materials.
The history of brazing
Brazing is the oldest method for joining metals, other than by mechanical means. Initially, the process was most popular for joining gold and silver base metals. Lead and tin, as well as alloys of gold-copper and silver-copper, were used as filler metals because of their low melting points. Copper hydrates and organic gums were added later
because of their reducing action, which helped to minimize oxidation and improve the cosmetic appearance of the joint. Metallic salts were also used.
Later, alloys of brass and copper were introduced as filler metals because of their ability to produce higher-strength joints in copper and steel structures, which were also able to withstand high temperatures.
As brazing technology advanced, manyother filler metals have evolved.
The term “brazing” can be applied to any process which joins metals (of the same or dissimilar composition)
through the use of heat and a filler metal with a melting temperature above 840° F (450° C), but below the melting point of the metals being joined. In furnace brazing, temperatures of 2050° to 2100° F (1120° to 1150° C) and above are not uncommon, especially when brazing stainless steels with nickel-based filler metals or carbon steel with copper filler metal. Other very high temperature brazing applications include molybdenum with pure nickel as the filler metal and cobalt with a cobalt alloy filler metal.
A successfully brazed joint often results in a metallurgical bond that is generally as strong or stronger than the base metals being joined. Modern brazing technology has extended the definition to include the bonding of metal to non-metallic substrates, including glass and refractory materials. However, this publication is limited to brazing of metals only,and, specifically, furnace brazing of metals.
How does brazing join materials? In furnace brazing, the parts or assemblies being joined are heated to the melting point of the filler metal being used. This allows the molten filler metal to flow via capillary action into the closefitting
surfaces of the joint and to form an alloy of the materials at the transition point upon solidification.
The base metals do not melt, but they can alloy with the molten filler metal by diffusion to form a metallurgical bond.
Because the metallurgical properties at the brazed joint may differ from those of the base metals, the selection of the appropriate filler metal is critical.
Depending on the desired properties of the application, the brazing operation can be used to impart a leaktight seal and/or structural strength, with excellent appearance characteristics, in addition to joining for the purpose of extending section length,e.g., in piping or tubing materials.
The history of brazing
Brazing is the oldest method for joining metals, other than by mechanical means. Initially, the process was most popular for joining gold and silver base metals. Lead and tin, as well as alloys of gold-copper and silver-copper, were used as filler metals because of their low melting points. Copper hydrates and organic gums were added later
because of their reducing action, which helped to minimize oxidation and improve the cosmetic appearance of the joint. Metallic salts were also used.
Later, alloys of brass and copper were introduced as filler metals because of their ability to produce higher-strength joints in copper and steel structures, which were also able to withstand high temperatures.
As brazing technology advanced, manyother filler metals have evolved.

Good
Bad
Related Content

Air Cooled clamp coil (half-open coil) for preheating gas pipeline

induction coating by air cooled clamp induction coil

Remove plastic coating from steel tubes by Induction Heating

Releasing Fixture by Induction Heating

U shape air cooled induction coil by UM-100C-HF for pipeline preheating

induction quenching with custom-design inductor

induction brazing diamond segment

induction brass soldering copper plate_2









Newest Comment
No Comment
Post Comment